Health & Scientific Research
Supported Projects
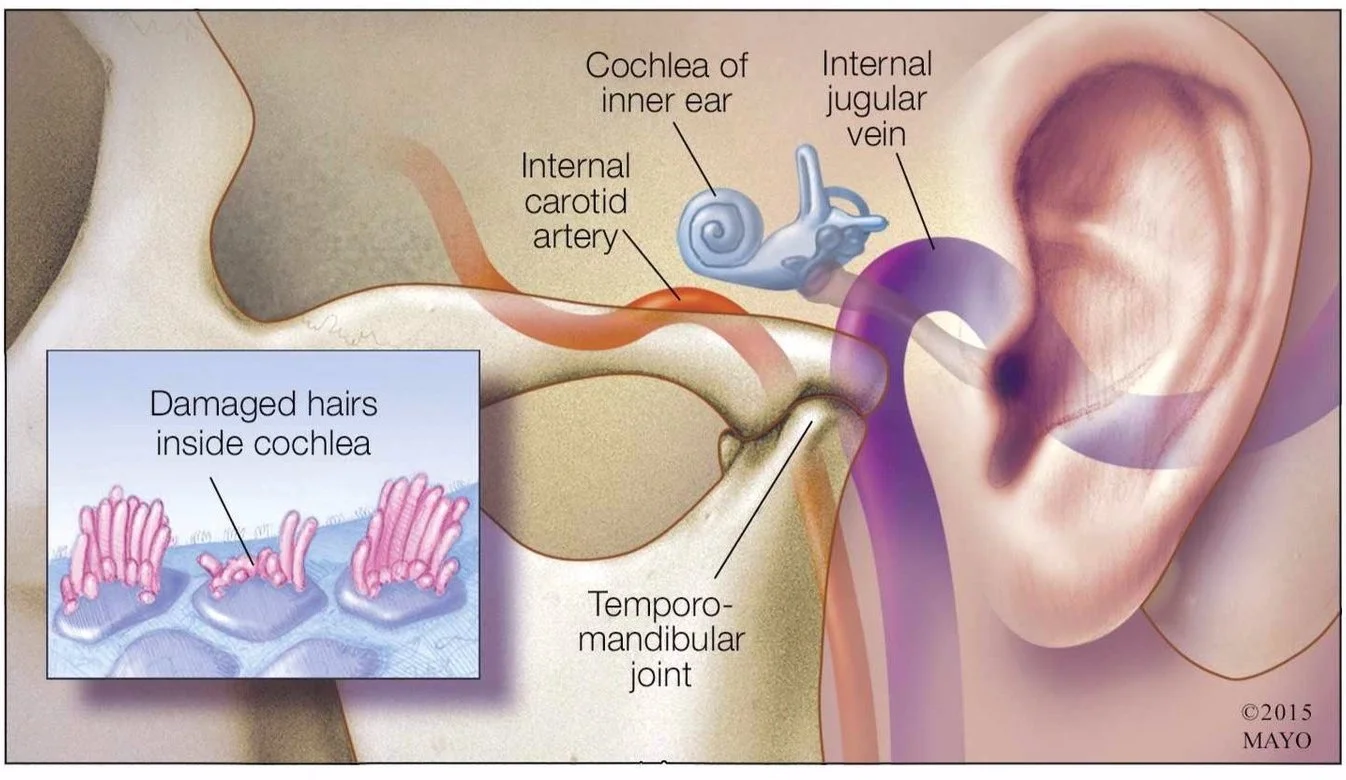
Tinnitus Research
In partnership with the Lauer family, Massachusetts Eye and Ear launched the Lauer Tinnitus Research Center in 2015 with the goal of advancing research to better understand and treat the debilitating condition of tinnitus. The Blakeley Foundation provides support to a new laboratory run by Daniel Polley in Eaton-Peabody Laboratories at Mass Eye & Ear, Boston.
Read More >
The Blakeley Foundation provides support to the Sleep Disorders Clinical Research Program, led by John Winkelman, MD, PhD, part of the Outpatient Psychiatry Division of Massachusetts General Hospital. The program is dedicated to clinical and investigative efforts in sleep disorders and the overlap of sleep medicine and psychiatric disorders. Read More >
Tinnitus: Characteristics, Causes, Mechanisms, and Treatments
Byung In Han, MDa; Ho Won Lee, MDb; Tae You Kim, MDc; Jun Seong Lim, MDd; Kyoung Sik Shin, MDe
aDo Neurology Clinic, Willis Medical Network, Daegu, Korea
Deptartment of bNeurology, Brain Science and Engineering Institute, Kyungpook National University School of Medicine, Daegu, Korea cWillis Hospital, Willis Medical Network, Busan, Korea
Department of dNeurology, Sung Ae Hospital, Seoul, Korea
eJeil Neurology Clinic, Willis Medical Network, Daejeon, Korea
J Clin Neurol 2009;5:11-19
Tinnitus-the perception of sound in the absence of an actual external sound-represents a symptom of an underlying condition rather than a single disease. Several theories have been proposed to explain the mechanisms underlying tinnitus. Tinnitus generators are theoretically located in the auditory pathway, and such generators and various mechanisms occurring in the peripheral au- ditory system have been explained in terms of spontaneous otoacoustic emissions, edge theory, and discordant theory. Those present in the central auditory system have been explained in terms of the dorsal cochlear nucleus, the auditory plasticity theory, the crosstalk theory, the somato- sensory system, and the limbic and autonomic nervous systems. Treatments for tinnitus include pharmacotherapy, cognitive and behavioral therapy, sound therapy, music therapy, tinnitus re- training therapy, massage and stretching, and electrical suppression. This paper reviews the char- acteristics, causes, mechanisms, and treatments of tinnitus.
J Clin Neurol 2009;5:11-19
Key Wordsaa tinnitus, discordant theory, tinnitus retraining therapy.
Received Revised Accepted
November 28, 2008 February 13, 2009 February 13, 2009
Correspondence
Byung In Han, MD
Do Neurology Clinic,
Willis Medical Network,
637 Jungang-dearo, Jung-gu,
Daegu 700-440, Korea,
Tel +82-53-252-2225
Fax +82-53-289-6502
E-mail han-byungin@hanmail.net
Introduction
Tinnitus is defined as a phantom auditory perception-it is a perception of sound without corresponding acoustic or me- chanical correlates in the cochlea.1 Tinnitus represents one of the most common and distressing otologic problems, and it causes various somatic and psychological disorders that in- terfere with the quality of life.2 A population-based study of hearing loss in adults aged 48 to 92 years found that tinnitus had a prevalence of 8.2% at baseline and an incidence of 5.7% during a 5-year follow-up.3 The prevalence of tinnitus increases with age.4
Tinnitus also represents a common symptom among chil- dren with hearing loss.5 Tinnitus is a subjective phenomenon that is difficult to evaluate objectively, with it being measur- ed, quantified, and described only based on the responses of patients. Although tinnitus can have many different causes, it most commonly results from otologic disorders, with the most common cause believed to be noise-induced hearing loss.6 The various therapeutic approaches to tinnitus have produced mixed results, and hence it is generally assumed that tinnitus has diverse physiological causes.7
Clinical Manifestations
Characteristics of tinnitus
The sound perceived by those with tinnitus can range from a quiet background noise to a noise that is audible over loud external sounds. Tinnitus is generally divided into two cate- gories: objective and subjective. Objective tinnitus is defined as tinnitus that is audible to another person as a sound ema- nating from the ear canal, whereas subjective tinnitus is au- dible only to the patient and is usually considered to be de- void of an acoustic etiology and associated movements in the cochlear partition or cochlear fluids. Many physicians use the term tinnitus to designate subjective tinnitus and the term so- matosound to designate objective tinnitus.6
The sounds associated with most cases of tinnitus have been described as being analogous to cicadas, crickets, winds, falling tap water, grinding steel, escaping steam, fluorescent lights, running engines, and so on. It is believed that these types of perception result from abnormal neuronal activity at a subcortical level of the auditory pathway.6,8
The pattern characterizing tinnitus is related to the library of patterns stored in auditory memory and also, via the lim-
Copyright c 2009 Korean Neurological Association 11
Tinnitus Review
bic system, associated with emotional states.9 The charac- teristics of tinnitus are generally unrelated to the type or sever- ity of any associated hearing impairment, and thus the latter of- fers little diagnostic value.6 Most tinnitus patients match their tinnitus to a pitch above 3 kHz.10 The tinnitus characterizing Meniere’s disease, described as roaring, matches a low-fre- quency tone that is usually from 125 to 250 Hz.11 However, tin- nitus in the advanced “burned-out” stage of Meniere’s dis- ease is often higher in pitch and tonal in quality.12
Most patients with both tinnitus and hearing loss report that the frequency of the tinnitus correlates with the severity and frequency characteristics of their hearing loss, and that the intensity of the tinnitus is usually less than 10 dB above the patient’s hearing threshold at that frequency.6 Some pa- tients who have central auditory processing disorders and have difficulties understanding speech in noise report experi- encing tinnitus even though their pure-tone audiometric thresholds are normal.8,13
Less prevalent forms of tinnitus, such as those involving well-known musical tunes or voices without understandable speech, occur among older people with hearing loss and are believed to represent a central type of tinnitus involving rever- beratory activity within neural loops at a high level of pro- cessing in the auditory cortex.8,14
Somatic tinnitus is a type of subjective tinnitus in which the frequency or intensity is altered by body movements such as clenching the jaw, turning the eyes, or applying pressure to the head and neck.15 Reports that tinnitus is louder upon awak- ening suggest the involvement of somatic factors, such as bruxism. Reports that tinnitus vanishes during sleep but re- turns within a few hours further suggest that psychosomatic factors, such as neck muscle contractions occurring in an upright position or jaw clenching, play etiological roles.16
Because objective tinnitus (which is audible to another person) represents the semantic opposite of subjective tinnit- us, a better nosological approach might be to use the term somatosound instead of objective tinnitus irrespective of whe- ther the sounds are audible to others, reserving the term tinn- itus for the perception of sound in the absence of any acous- tic source. Thus, “tinnitus” would describe cases previously diagnosed as subjective tinnitus.6 Objective tinnitus might be vascular or mechanical in origin. Objective tinnitus of vascul- ar origin could be a referred bruit from stenosis in the carotid or vertebrobasilar system. Objective mechanical tinnitus is due to abnormal muscular contraction of the nasopharynx or mid- dle ear, as can occur in palatal myoclonus.17 Pulsatile tinnitus can also manifest subjectively as an increased awareness of blood flow in the ear. Indeed, the cause of somatosensory pul- satile tinnitus syndrome is not vascular, with the syndrome de- riving from cardiac-synchronous somatosensory activation of
the central auditory pathway or the failure of somatosensory- auditory central nervous system (CNS) interactions to sup- press cardiac somatosounds.18 Pulsatile tinnitus superimpos- ed on steady tinnitus could result from the pulsation of blow flow with the spiral capillary of the basilar membrane.19
Associated symptoms
The most common associated symptoms or subjective dis- comforts involve concentration difficulties, insomnia, and de- creased speech discrimination.20 The annoyance of tinnitus is not correlated with the acoustic characteristics, but there is a significant correlation with psychological symptoms.21 The difference between simply perceiving tinnitus and being an- noyed or distressed by it depends exclusively on the activation of the limbic and autonomic nervous systems.8 Most patients with significant tinnitus have difficulty falling asleep due to the accompanying anxiety, which also causes difficulties in returning to sleep during periods of wakefulness during the night.8
There is pronounced neuronal activity in the auditory path- ways during sleep due to the auditory system continuously monitoring the sound environment.8 Common detrimental ac- tivities and/or conditions include noise exposure, being locat- ed in a quiet place, emotional stress, loss of sleep, and physi- cal exhaustion.22 Annoyance, depression, and interference with sleep are more common and the tinnitus is louder in patients with Meniere’s disease than in those with tinnitus deriving from other etiologies.22 Furthermore, the successful control of vertigo in patients with Meniere’s disease can lead to them focusing more on their tinnitus and hence becoming more distressed by this condition.12
The strength of the reaction to tinnitus is therefore deter- mined by its significance and by past experience-the actual intensity and characteristics of the sound are of secondary im- portance.23
Natural course
Noise-induced tinnitus can be acute or chronic. Acute tinnitus can last from a few minutes to a few weeks after noise expo- sure.24 In some cases, tinnitus has a gradual onset and several years can pass before an intermittent, low-intensity tinnitus becomes bothersome.25 Spontaneous remission by natural ha- bituation is experienced by more than three-quarters of suffer- ers. Habituation occurs within the CNS, whereas adaptation involves a peripheral sensory organ.8 For those in whom the condition worsens, the tinnitus intensity increases over time but its pitch tends to remain stable.22 If tinnitus persists for more than 2 years, it is considered permanent and irreversi- ble.26 However, chronicity is not associated with a favorable response to treatment.27
12 J Clin Neurol 2009;5:11-19
Causes and Pathophysiology
Causes
Tinnitus does not represent a disease itself but instead is a symptom of a variety of underlying diseases. Otologic causes include noise-induced hearing loss, presbycusis, otosclerosis, otitis, impacted cerumen, sudden deafness, Meniere’s disease, and other causes of hearing loss. Neurologic causes include head injury, whiplash, multiple sclerosis, vestibular schwan- noma (commonly called an acoustic neuroma), and other cer- ebellopontine-angle tumors. Infectious causes include otitis media and sequelae of Lyme disease, meningitis, syphilis, and other infectious or inflammatory processes that affect hearing. Tinnitus is also a side effect of some oral medications, such as salicylates, nonsteroidal anti-inflammatory drugs, aminoglyco- side antibiotics, loop diuretics, and chemotherapy agents (e.g., platins and vincristine). Temporomandibular-joint dysfunction and other dental disorders can also cause tinnitus. However, in many cases no underlying physical cause is identifiable.28 For many years, hearing loss has been understood to be the most common cause of tinnitus,29 and population-based data indi- cate that excessive noise exposure represents the second most common cause of tinnitus. However, about 40% of patients cannot identify any cause associated with tinnitus onset.26
Any pathologic lesion in the auditory pathway or any re- duction in auditory nerve function has the potential to pro- duce tinnitus.19 The location of the hearing problem (i.e., in the middle ear or in the inner ear) and the otologic disorder caus- ing the hearing loss do not appear to influence the etiologic potential.6 Interestingly, most patients with tinnitus complain about a sensation of fullness or blockage in the middle ear, suggesting a problem with middle ear pressure or increased impedance of the ossicular chain.30
Unilateral high-frequency hearing loss combined with poor speech discrimination suggests the presence of a tumor, usual- ly a vestibular schwannoma/acoustic neuroma or a meningio- ma.28 Bilateral subjective tinnitus requires assessment of hear- ing and can be associated with presbycusis, noise-induced hearing loss, endolymphatic hydrops, and a vascular labyrin- thine lesion.21 However, most cases of unilateral tinnitus are not associated with life-threatening otologic disease.6
Trigger factors
Small temporary changes in the outer hair cells (OHCs) fol- lowing noise exposure can trigger the emergence of tinnitus by increasing the gain of the central auditory system.8 In gen- eral, tinnitus represents a threshold phenomenon for which any one factor, such as chronic progressive hearing loss, is insuf- ficient to elicit its emergence-two or more trigger factors (i.e., psychosocial stress, noise exposure, and somatic factors) can
Han BI et al.
act synergistically to produce symptomatic tinnitus.15 About 75% of new cases are related to emotional stress as the trigger factor rather than to precipitants involving cochlear lesions.8
Pathophysiology
Tinnitus represents a symptom of diverse pathologies. It is proposed that all levels of the nervous system are, to varying degrees, involved in tinnitus manifestation.1,31
Peripheral auditory system
Spontaneous otoacoustic emissions
Spontaneous otoacoustic emissions (SOAEs), first discover- ed by Kemp,32 are small acoustic signals presumed to be ge- nerated by the electromotile activity of the OHCs of the coch- lea and propagated into the external auditory canal.33 SOAEs produced by the cochlea can be perceived as tinnitus.34 SOAEs are usually inaudible, but they can become audible due to instability.35 These atypical SOAEs are much more prevalent in the higher frequency range and can appear at sound pres- sure levels up to 55 dB SPL in the ear canal.36 Tinnitus due to SOAEs is mild and is more common in subjects with normal hearing and in those with only middle ear disorders.37 SOAEs decrease as hearing loss progresses, and hence these otoa- coustic emissions are not likely to cause tinnitus when a hearing loss of 35 dB or more is present.38 However, SOAEs cannot fully explain the mechanism of tinnitus since aspirin largely abolishes SOAEs without improving tinnitus.39
Edge theory
Edge theory, also known as contrast theory, proposes that tinnitus is induced by increased spontaneous activity in the edge area,40 which represents a transition from OHCs in the organ of Corti with relatively normal morphology and func- tion on the apical (i.e., low-frequency) side of a lesion to OHCs toward the basal side that are missing or have a pathologic appearance and poor functionality.19 Edge theory can be ex- plained by discordant theory.9
Discordant theory
According to discordant theory, tinnitus is induced by the dis- cordant dysfunction of damaged OHCs and intact inner hair cells (IHCs) of the organ of Corti. Intense noise and ototoxic agents initially damage OHCs in the basal turn of the cochl- ea, and subsequently, if continued or repeated, affect IHCs- this is due to IHCs being more resistant to such damage.9 IHCs are the receptor cells for sound transduction, and almost all afferent fibers in the auditory nerve (95%) innervate IHCs.8 In contrast, OHCs work as mechanical amplifiers, enhancing weak sounds by providing up to 50 dB, which can be evalu-
www.thejcn.com 13
Tinnitus Review
ated by measuring otoacoustic emissions.8 In almost all situ- ations OHCs are damaged more than IHCs, which results in the disinhibition of neurons in the dorsal cochlear nuclei (DCNs).8 Spontaneous activity is increased when neurons in the DCN receive excitation from IHCs but not from the dam- aged OHCs, and this is perceived as tinnitus.8 Normally there is a small gap between the top of the cilia of the IHCs and the bottom of the tectorial membrane, but in the area in which OHCs are affected but IHCs are intact, the tectorial mem- brane might touch the IHC cilia, thus causing the IHCs to de- polarize.41 The OHCs normally recover with a few days, but this can be delayed for up to a few months.42,43 Therefore, it is hypothesized that tinnitus represents a consequence of a cen- tral gain adaptation mechanism when the auditory system is confronted with a hearing loss.44 Discordant theory explains why many individuals with tinnitus have normal hearing if there is only partial damage to OHCs, since up to 30% of OHCs can be damaged without inducing hearing loss.45 OHCs die at a rate of approximately 0.5% per year beginning during the first years of life, and OHC-induced hearing loss does not usually appear before the end of the fifth decade of life.8 Discordance is absent in totally deaf individuals with comple- te damage to both OHCs and IHCs, and hence tinnitus is not induced. If there is increased gain within the CNS, tinnitus is present even in deaf subjects.23 Similarly, noise-induced tin- nitus is caused by discordant damage between OHCs and IHCs.41 Two types of noise-induced tinnitus have been iden- tified: tonal and complex. Tonal tinnitus results from discor- dant dysfunction of OHCs and IHCs manifesting in a single area, whereas complex tinnitus results from multiple areas of discordance.46 When patients clearly have the central type of tinnitus, such as after transection of the auditory nerve, the OHC concept is not applicable and alternative mechanisms need to be considered.23
Central auditory system
The dorsal cochlear nucleus
The DCN has been implicated as a possible site for the gen- eration of tinnitus-related signals owing to its tendency to become hyperactive following exposure to tinnitus-inducing agents such as intense sound and cisplatin.47 OHC damage triggers plastic readjustments in the DCN, resulting in DCN hyperactivity.48 It is hypothesized that a reduction in auditory- nerve input leads to disinhibition of the DCN and an increase in spontaneous activity in the central auditory system, which manifests as tinnitus.49 This mechanism could explain the tem- porary ringing sensation that can follow exposure to loud sound.50 The plastic readjustments in the DCN are slow and lead to tinnitus with a delayed onset. IHC damage prevents
hyperactivity in the DCN.51
Auditory plasticity theory
According to auditory plasticity theory, damage to the cochlea enhances neural activity in the central auditory pathway.52 Auditory plasticity emerges as a consequence of the aberrant pathway, and tinnitus might be considered to be the auditory system analog to phantom limb sensations in amputees.53 Tin- nitus might be generated in the temporal lobe in the auditory association cortex54 and inferior colliculus.55 The ability of some individuals to modulate tinnitus by performing voluntary so- matosensory or motor actions is probably attributable to plastic changes involving the development of aberrant connections between the auditory and sensory-motor systems in the brains of these patients.56
Crosstalk theory
According to crosstalk theory, when auditory nerve fibers are intact and some other cranial nerves are damaged, artificial synapses (called crosstalk) can develop between individual au- ditory nerve fibers, resulting in the phase-locking of the spon- taneous activity of groups of auditory neurons. In the ab- sence of external sounds, this creates a neural pattern that re- sembles patterns evoked by actual sounds.57 These cranial nerves are sensitive to compression at the root entry zone, where they are covered by myelin. Nerve compression causes crosstalk between nerve fibers, and the breakdown of the myelin insulation of the nerve fibers establishes ephaptic cou- pling between them. This notion is applied to the cochlear- vestibular nerve, which is covered by central myelin for most of its length and hence is vulnerable to compression from blood vessels or tumors impinging upon the nerve (e.g., ves- tibular schwannoma). Such compression and consequent ep- haptic coupling might lead to tinnitus if synchronization of the stochastic firing in the cochlear nerve is perceived as sound.57
Somatosensory system
The activity of the DCN is also influenced by stimulation of nonauditory structures;47 however, the somatosensory system is the only nonauditory sensory system that appears to be re- lated to tinnitus (e.g., in temporomandibular-joint syndrome and whiplash).49 Somatic tinnitus can develop from activation of latent oto-somatic interaction.16 Somatic (craniocervical) tinni- tus, like otic tinnitus, is caused by disinhibition of the ipsilater- al DCN, which is mediated by nerve fibers whose cell bodies lie in the ipsilateral medullary somatosensory nuclei. These neurons receive inputs from the nearby spinal trigeminal tract, the fasciculus cuneatus, and the facial, vagal, and glossophar- yngeal nerve fibers innervating the middle and external ears.49
Pain signals from the cochlea carried by the cochlear C fi-
14 J Clin Neurol 2009;5:11-19
bers could also be interpreted by the CNS as tinnitus.58 It is fur- ther hypothesized that somatic tinnitus is due to central cross- talk within the brain, because certain head and neck nerves enter the brain near regions known to be involved in hearing.57
Limbic and autonomic nervous systems
The aforementioned theories cannot explain why some people suffer from tinnitus while others do not. More than 80% of those perceiving tinnitus for the first time do not associate the sound with any negative meaning and experience spontane- ous habituation. However, if the first perception of tinnitus induces high levels of annoyance or anxiety by association with unpleasant stimuli or with periods of stress and anxiety, tinnitus mightlead to high levels of annoyance or anxiety. At the unconscious level, tinnitus can increase progressively with- out the patient being aware, resulting in enhanced activity in the limbic and autonomic nervous systems. In such situa- tions, tinnitus emerges as a clinically significant problem.8
Treatments
Tinnitus treatments can be divided into two categories: 1) those aimed at directly reducing the intensity of tinnitus and 2) those aimed at relieving the annoyance associated with tin- nitus. The former include pharmacotherapy and electrical sup- pression,59 and the latter include pharmacotherapy, cognitive and behavioral therapy, sound therapy, habituation therapy,60 massage and stretching, and hearing aids.
Pharmacotherapy
Extensive reviews of randomized clinical trials have revealed that only nortriptyline, amitriptyline, alprazolam, clonazepam, and oxazepam are more beneficial than placebo.59,61 Dobie et al. reported that nortriptyline were statistically superior to placebo, although placebo was also effective.62 Podoshin et al. reported that amitriptyline was superior to placebo with re- spect to sleep disturbance and interference with activities.63 Johnson et al. reported that alprazolam was more effective than placebo in reducing tinnitus intensity.64 Lechtenberg and Shulman noted that clonazepam and oxazepam were effective in more than 50% of tinnitus cases.65 However, when patients stopped taking either of these drugs, tinnitus recurred to its prior level or a worse level.65 The only medication providing a reliable reduction of tinnitus is intravenous lidocaine, and there is a close association between the effects of lidocaine and oral carbamazepine.66 Intravenous lidocaine produces a change in the neural activity in the right temporal lobe in the auditory association cortex.54 Unfortunately, lidocaine cannot be used clinically because it must be injected, its effects are of short duration, and it frequently produces adverse side
Han BI et al.
effects.61 Tocainide, an oral antiarrhythmic drug closely re- lated to lidocaine, is not beneficial.67 Tinnitus due to SOAEs can be diminished by aspirin.68 A recent 3-month randomized clinical trial involving 50 patients found that acamprosate, a drug used in the treatment of alcoholism, was more benefi- cial than placebo.69 Flecainide, mexiletine, betahistine, carba- mazepine, ginko extract, amylobarbiturate, baclofen, lamotri- gine, misoprostol, zinc, cinnarizine, flunarizine, caroverine, ep- erisone, and melatonin are no more beneficial than placebo.59 Diazepam and flurazepam significantly change the tinnitus intensity.61
Cognitive and behavioral therapy
Cognitive therapy focuses on how one thinks about tinnitus and on the avoidance of negative ideation, whereas behav- ioral therapy uses the systematic desensitization approach that is applied to many phobias.70 Cognitive therapy involves teach- ing patients to cope with their tinnitus by replacing negative thinking with more positive thinking. Cognitive therapy in- cludes counseling and cognitive restructuring. Counseling should include 1) informing patients that it is unlikely that their annoyance with tinnitus will improve dramatically, 2) inform- ing patients about the usefulness of tinnitus self-help groups, 3) helping patients to minimize the time devoted to activities and/or conditions in which the tinnitus intensity is increased and to maximize the time devoted to activities and/or condi- tions in which the tinnitus intensity is decreased, and 4) stress- ing the avoidance of noise exposure, since noise-induced hearing loss and tinnitus are related.22 Cognitive restructur- ing involves changing thoughts associated with tinnitus. In this context, patients are encouraged to accept the idea that tin- nitus does not deserve all the attention it gets.71 Behavioral therapy focuses on positive imagery, attention control, and re- laxation training.70 Positive imagery involves focusing thou- ghts on something pleasant, thereby diverting thoughts from tinnitus. Patients begin with pleasant visual images (e.g., lying on a beach) and auditory images (e.g., the sound of waves or wind through the leaves).70 Attention control involves moving attention away from the tinnitus when it is bothersome. This process might begin by placing two pictures next to one ano- ther and then presenting two acoustic stimuli (e.g., a fan noise and conversational speech) emanating from an adjacent room. Next, a picture and the tinnitus are paired, followed by the pairing of a thought and the tinnitus.70 Relaxation training uses a guided protocol to teach participants to apply progressive muscle relaxation, involving tensing and relaxing the arms, face, neck, shoulders, abdomen, legs, and feet.70
Sound therapy
Sound therapy uses sounds found in natural settings, including
www.thejcn.com 15
Tinnitus Review
those associated with streams, rain, waterfalls, and wind, to decrease the strength of the tinnitus-related neuronal activity within the auditory system.72 To this end, the background neuronal activity in the auditory system is increased by expos- ing the patient to a low-level, continuous, neutral sound23 that is nonintrusive, not annoying, and easy to ignore. Such a sound should not be meaningful, pleasant, or arousing in a way that attracts attention, making listening to a television, the radio, or music unsuitable.8 Neutral sounds should be stable and not overwhelming; therefore, the sounds of waves are not recom- mended.23 Some patients are distracted by the sounds of bird calls, crickets, or thunderstorms, and hence care is required when applying these sounds.72 Sound therapy can employ var- ious sound sources, such as table-top sound machines and compact disc players. The sound intensity should be at or be- low the level at which the patient can perceive the tinnitus and the external sound separately.23 The sound must be ap- plied bilaterally to avoid asymmetrical stimulation of the au- ditory system, since stimulating only one side in unilateral tinnitus frequently results in a shift of the perceived location of the tinnitus to the opposite side due to strong interactions within the auditory pathways. Occlusion with ear plugs should be minimized by using open-ear molds to allow normal ac- cess of environmental sounds to the ear.8 Applying sound therapy during the night can be helpful for individuals with- out sleep problems because the auditory pathways are fully active up to the level of the inferior colliculi during sleep.23 Based on a report that the continuous sound exposure in- creases blood flow to the inner ear of rat,73 sound therapy mi- ght affect the physiology of human cochlea.
Hearing aids
Hearing aids represent another form of sound therapy that is usually beneficial to tinnitus patients with significant hearing loss.72 Hearing aids are designed to improve the audibility of speech and to amplify ambient sounds. Amplification of spe- ech diverts attention away from tinnitus, and amplification of other ambient sounds serves to partially mask tinnitus. Hear- ing aids are not appropriate for those with hearing loss con- fined to above 6 kHz, because most hearing aids have limit- ed high-frequency amplification abilities.74 The use of hear- ing aids can permanently reduce the neural activity respon- sible for tinnitus generation and perception,72 and usually re- presents the first intervention for patients with hearing im- pairment.74 Many hearing-impaired patients have normal or near-normal hearing at low frequencies, and common environ- mental sounds contain a significant amount of energy below 200 Hz, which provides constant sound stimulation and thus helps to prevent difficulties associated with increased gain in the auditory system. Therefore, it is crucial to fit hearing aids
with open molds in the external ear to avoid blocking these low frequencies.8
Music therapy
Music therapy is a desensitization method that utilizes music that has been spectrally modified according to the hearing char- acteristics of each patient to allow the masking of tinnitus and to facilitate relaxation at a comfortable listening level.75 Music directly affects the limbic system, bypassing the slower linguis- tically based processing in the auditory cortex.75 Hearing thresh- olds decline substantially above 3 kHz among many tinnitus pa- tients, and hence the spectral modification should involve reduc- ing the energy of lower frequency components of the music.75
Tinnitus retraining therapy
Tinnitus retraining therapy (TRT) is a form of habituation therapy designed to help tinnitus sufferers. TRT mainly targets nonauditory systems, particularly the limbic and autonomic nervous systems, and is based on the assumption that tinnitus represents a side effect of the normal compensatory mecha- nisms in the brain. TRT uses naturally occurring mechanisms of plasticity in the brain to achieve habituation to the physio- logical reactions to tinnitus and, subsequently, to achieve ha- bituation to the very perception of tinnitus.8 Habituation is typ- ically achieved by repeating the sensory stimulus. However, this method cannot be directly applied to tinnitus because it is impossible to eliminate the reactions of the autonomic nervous system that act as a negative reinforcement. Therefore, TRT involves decreasing both the stimulus and the reinforcement, even though these remain present.8
TRT consists of two components: retraining counseling and sound therapy. Retraining counseling aims to help patients to think of their tinnitus as a type of neutral sound.8 Neutraliz- ing tinnitus is achieved by showing the patient that tinnitus is not associated with threatening pathology.23 The creation of positive associations with tinnitus represents an additional way of neutralizing tinnitus. Descriptions such as screeching, tear- ing, and steam jets should be replaced by benign, more peace- ful descriptions, such as “music of the brain”.8 Sound ther- apy aims at facilitating habituation at an unconscious level by decreasing the strength of the signal. The addition of sound decreases the difference between tinnitus and background sounds.8 However, TRT requires about 18 months to achieve observable stable effects, and this time-consuming treatment does not achieve satisfactory results in some patients. TRT requires patience and discipline from both the patient and a knowledgeable and experienced professional.28
Massage and stretching
Massage and stretching of the neck and masticatory muscles
16 J Clin Neurol 2009;5:11-19
have been associated with significant improvement in tinni- tus.59 Patients with somatic tinnitus can have symptoms of cervical spine disorders, including head, neck, and shoulder pain as well as limitations in sideways bending and rotation. Treating jaw and neck disorders has beneficial effects on tin- nitus. Injecting lidocaine into jaw muscles, such as the lateral pterygoid, also reduces tinnitus.76
Electrical suppression
Electrical stimulation of the cochlea with trains of pulses at 5,000 pulses per second can substantially or completely sup- press tinnitus with either no perception or only a transient perception of the stimulus. Stimulus with electrical pulses at such a high rate restores spontaneous-like patterns of spike ac- tivity in the auditory nerve, which could explain how it sup-
77 presses tinnitus.
Transcutaneous electrical nerve stimulation of areas of skin close to the ear increases the activation of the DCN via the so- matosensory pathway and could augment the inhibitory role played by this nucleus on the CNS, thereby ameliorating tin- nitus.78
Conclusion
Tinnitus frequently represents a symptom of an associated disease process. Recent research has employed state-of-the-art imaging and measurement technology to examine tinnitus-re- lated activity in the ear, auditory nerve, and auditory tracts of the brain. These studies have increasingly focused on explor- ing putative brain-related mechanisms. The complexity of the changes in the nervous system associated with tinnitus might explain why this condition has proved so resistant to treat- ment.28 Although numerous therapeutic modalities have been applied, no consensus regarding effective therapeutic agents has emerged. At times, no treatment represents the better alter- native, mandating that clinicians are able to placate patients without resorting to unnecessary prescriptions.28 Although treat- ment does not necessarily relieve tinnitus, accurate diagnosis and treatment are important for reduing the annoyance asso- ciated with tinnitus and for preventing additional disability. Furthermore, many randomized clinical trials have found strong placebo effects that have been partly attributed to re- sponses to attention.
Nevertheless, counseling represents an essential part of treat- ment, regardless of the management approach adopted for a particular patient. An informed explanation of tinnitus, togeth- er with reassurance, improves the condition of most patients over time. For those with persistent tinnitus, cognitive and behavioral therapy, augmented by pharmacologic interven- tion, might represent the most promising treatment regimen.
Han BI et al.
Most importantly, a strong doctor-patient relationship under- pins successful management and high levels of satisfaction among patients.
REFERENCES
1. Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 1990;8:221-254.
2. Yetiser S, Tosun F, Satar B, Arslanhan M, Akcam T, Ozkaptan Y. The role of zinc in management of tinnitus. Auris Nasus Larynx 2002;29: 329-333.
3. Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BE, Tweed TS. Prevalence and 5-year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J Am Acad Audiol 2002;13: 323-331.
4. Daniell WE, Fulton-Kehoe D, Smith-Weller T, Franklin GM. Occupa- tional hearing loss in Washington state, 1984-1991: II. Morbidity and associated costs. Am J Ind Med 1998;33:529-536.
5. Coelho CB, Sanchez TG, Tyler RS. Tinnitus in children and associ ated risk factors. Prog Brain Res 2007;166:179-191.
6. Dobie RA. Overview: suffering from tinnitus. In: snow JB. Tinnitus: theory and management. Ontario: BC Decker Inc, 2004;1-7.
7. Baguley DM. Mechanisms of tinnitus. Br Med Bull 2002;63:195-212. 8. Jastreboff PJ, Hazell JW. Tinnitus Retraining Therapy. New York:
Cambridge University Press, 2004.
9. Jastreboff PJ, Hazell JW. A neurophysiological approach to tinnitus:
clinical implications. Br J Audiol 1993;27:7-17.
10. Baguley DM, Williamson CA, Moffat DA. Treating tinnitus in patients
with otologic conditions. In: Tyler RS. Tinnitus treatment. New York:
Thieme, 2006;41-50.
11. Douek E, Reid J. The diagnostic value of tinnitus pitch. J Laryngol
Otol 1968;82:1039-1042.
12. Vernon J, Johnson R, Schleuning A. The characteristics and natural
history of tinnitus in Meniere’s disease. Otolaryngol Clin North Am
1980;13:611-619.
13. Higson JM, Haggard MP, Field DL. Validation of parameters for as-
sessing Obscure Auditory Dysfunction-robustness of determinants of OAD status across samples and test methods. Br J Audiol 1994;28: 27-39.
14. Berrios GE. Musical hallucinations: a statistical analysis of 46 cases. Psychopathology 1991;24:356-360.
15. Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog Brain Res 2007;166:107-123.
16. Levin RA. Somatic tinnitus. In: Snow JB. Tinnitus: theory and ma- nagement. Ontario: BC Decker Inc, 2004;108-124.
17. Troost BT, Waller MA. Hearing loss and tinnitus without dizziness or vertigo. In: Bradley WG, Daroff RB, Fenichel GM, Marsden CD. Neu- rology in clinical practice. 2nd ed. Newton: Butterworth-Heinemann, 1996;239-241.
18. Levine RA, Nam EC, Melcher J. Somatosensory pulsatile tinnitus syn- drome: somatic testing identifies a pulsatile tinnitus subtype that impli- cates the somatosensory system. Trends Amplif 2008;12:242-253.
19. Nuttal AL, Meikle MB, Trune DR. Peripheral process involved in tin- nitus. In: Snow JB. Tinnitus: theory and management. Ontario: BC Deck- er Inc, 2004;52-68.
20. Axelsson A, Sandh A. Tinnitus in noise-induced hearing loss. Br J Audiol 1985;19:271-276.
21. Luxon LM. Tinnitus: its causes, diagnosis, and treatment. BMJ 1993; 306:1490-1491.
22. Stouffer JL, Tyler RS, Kileny PR, Dalzell LE. Tinnitus as a function of duration and etiology: counselling implications. Am J Otol 1991; 12:188-194.
23. Jastreboff PJ, Jastreboff MM. Tinnitus retraining therapy. In: Snow J. Tin- nitus: theory and management. Ontario: BC Decker Inc, 2004;295-309.
www.thejcn.com 17
Tinnitus Review
Kaltenbach, Zhang J, Zacharek MA. Neural correlates of tinniuts: acute noise-induced tinnitus. In: Snow J. Tinnitus theory and manage- ment. Ontario: BC Decker Inc, 2004;142.
Erlandsson SI, Hallberg LR, Axelsson A. Psychological and audio- logical correlates of perceived tinnitus severity. Audiology 1992;31: 168-179.
Henry JA. Audiologic Assessment. In: Snow J. Tinnitus: theory and management. Ontario: BC Decker Inc, 2004;220-236.
Sanchez TG, Balbani AP, Bittar RS, Bento RF, Câmara J. Lidocaine test in patients with tinnitus: rationale of accomplishment and relation to the treatment with carbamazepine. Auris Nasus Larynx 1999;26: 411-417.
Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med 2002; 347:904-910.
Leske MC. Prevalence estimates of communicative disorders in the U.S. Language, hearing and vestibular disorders. ASHA 1981;23:229- 237.
Ren YF, Isberg A. Tinnitus in patients with temporomandibular joint internal derangement. Cranio 1995;13:75-80.
Hazell JW, Jastreboff PJ. Tinnitus. I: Auditory mechanisms: a model for tinnitus and hearing impairment. J Otolaryngol 1990;19:1-5.
Kemp DT. Stimulated acoustic emissions from within the human au- ditory system. J Acoust Soc Am 1978;64:1386-1391.
Brownell WE. Outer hair cell electromotility and otoacoustic emis- sions. Ear Hear 1990;11:82-92.
Huang ZW, Luo Y, Wu Z, Tao Z, Jones RO, Zhao HB. Paradoxical enhancement of active cochlear mechanics in long-term administra- tion of salicylate. J Neurophysiol 2005;93:2053-2061.
Penner MJ. Audible and annoying spontaneous otoacoustic emissions. A case study. Arch Otolaryngol Head Neck Surg 1988;114:150-153.
Mathis A, Probst R, De Min N, Hauser R. A child with an unusually high-level spontaneous otoacoustic emission. Arch Otolaryngol Head Neck Surg 1991;117:674-676.
Kemp DT. Physiologically active cochlear micromechanics--one sou- rce of tinnitus. Ciba Found Symp 1981;85:54-81.
Probst R, Lonsbury-Martin BL, Martin GK, Coats AC. Otoacoustic emissions in ears with hearing loss. Am J Otolaryngol 1987;8:73-81.
Penner MJ, Burns EM. The dissociation of SOAEs and tinnitus. J Speech Hear Res 1987;30:396-403.
Kiang NY, Moxon EC, Levine RA. Auditory-nerve activity in cats with normal and abnormal cochleas. In: Sensorineural hearing loss. Ciba Found Symp 1970:241-273.
Baguley DM. Mechanisms of tinnitus. Br Med Bull 2002;63:195-212.
Cardinaal RM, de Groot JC, Huizing EH, Veldman JE, Smoorenburg GF. Cisplatin-induced ototoxicity: morphological evidence of spon- taneous outer hair cell recovery in albino guinea pigs? Hear Res 2000;
144:147-156.
Emmerich E, Richter F, Reinhold U, Linss V, Linss W. Effects of
industrial noise exposure on distortion product otoacoustic emissions (DPOAEs) and hair cell loss of the cochlea--long term experiments in awake guinea pigs. Hear Res 2000;148:9-17.
Parra LC, Pearlmutter BA. Illusory percepts from auditory adapta- tion. J Acoust Soc Am 2007;121:1632-1641.
Chen GD, Fechter LD. The relationship between noise-induced hear- ing loss and hair cell loss in rats. Hear Res 2003;177:81-90.
Jastreboff PJ. The neurophysiological model of tinnitus. In: Snow J. Tinnitus: theory and management. Ontario: BC Decker Inc, 2004;96- 107.
Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenom- enon and its possible neural underpinnings in the dorsal cochlear nu- cleus. Hear Res 2005;206:200-226.
Kaltenbach JA, Zhang J, Zacharek MA. Neural correlates of tinnitus. In: Snow J. Tinnitus: theory and management. Ontario: BC Decker Inc, 2004;141-161.
Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear
18 J Clin Neurol 2009;5:11-19
nucleus hypothesis. Am J Otolaryngol 1999;20:351-362.
50. Schreiner CE, Cheung SW. Cortical plasticity and tinnitus. In: Snow JB. Tinnitus: Theory and management. Ontario: BC Decker Inc. 2004;
189-204.
51. Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural
activity in the dorsal cochlear nucleus after intense sound exposure.
Hear Res 2000;147:282-292.
52. Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity fol-
lowing cochlear damage. Hear Res 2000;147:261-274.
53. Lockwood AH, Salvi RJ, Burkard RF, Galantowicz PJ, Coad ML, Wack DS. Neuroanatomy of tinnitus. Scand Audiol Suppl 1999;51:
47-52.
54. Reyes SA, Salvi RJ, Burkard RF, Coad ML, Wack DS, Galantowicz
PJ, et al. Brain imaging of the effects of lidocaine on tinnitus. Hear
Res 2002;171:43-50.
55. Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla
inferior colliculus associated with chronic and acute cochlear damage.
Hear Res 2002;168:238-249.
56. Pinchoff RJ, Burkard RF, Salvi RJ, Coad ML, Lockwood AH. Mod-
ulation of tinnitus by voluntary jaw movements. Am J Otol 1998;19:
785-789.
57. Møller AR. Pathophysiology of tinnitus. Ann Otol Rhinol Laryngol
1984; 93:39-44.
58. Moller AR. Similarities between severe tinnitus and chronic pain. J
Am Acad Audiol 2000;11:115-124.
59. Dobie RA. A review of randomized clinical trials in tinnitus. Laryn-
goscope 1999;109:1202-1211.
60. Tyler RS. Neurophysiological models, psychological models, and treat-
ments for tinnitus. In: Tyler RS. Tinnitus treatment. New York: Thieme,
2006;1-22.
61. Murai K, Tyler RS, Harker LA, Stouffer JL. Review of pharmaco-
logic treatment of tinnitus. Am J Otol 1992;13:454-464.
62. Dobie RA, Sakai CS, Sullivan MD, Katon WJ, Russo J. Antidepres- sant treatment of tinnitus patients: report of a randomized clinical trial
and clinical prediction of benefit. Am J Otol 1993;14:18-23.
63. Podoshin L, Ben-David Y, Fradis M, Malatskey S, Hafner H. Idio- pathic Subjective Tinnitus Treated by Amitriptyline Hydrochloride/
Biofeedback. Int Tinnitus J 1995;1:54-60.
64. Johnson RM, Brummett R, Schleuning A. Use of alprazolam for re-
lief of tinnitus. A double-blind study. Arch Otolaryngol Head Neck
Surg 1993;119:842-845.
65. Lechtenberg R, Shulman A. Benzodiazepines in the treatment of tin-
nitus. J Laryngol Otol Suppl 1984;9:271-276.
66. Sanchez TG, Balbani AP, Bittar RS, Bento RF, Câmara J. Lidocaine
test in patients with tinnitus: rationale of accomplishment and rela- tion to the treatment with carbamazepine. Auris Nasus Larynx 1999; 26:411-417.
67. Vermeij P, Hulshof JH. Dose finding of tocainide in the treatment of tinnitus. Int J Clin Pharmacol Ther Toxicol 1986;24:207-212.
68. Penner MJ. Aspirin abolishes tinnitus caused by spontaneous otoac- oustic emissions. A case study. Arch Otolaryngol Head Neck Surg 1989;115:871-875
69. Azevedo AA, Figueiredo RR. Tinnitus treatment with acamprosate: double-blind study. Braz J Otorrinolaringol. 2005;71: 618-623.
70. Tyler RS, Noble W, Preece J, Dunn CC, Witt SA. Psychological treatments for tinnitus. In: Snow J. Tinnitus: theory and management. Ontario: BC Decker Inc, 2004;314-325.
71. Andersson G, Kaldo V. Cognitive-behavioral therapy with applied relaxation. In: Tyler RS. Tinnitus treatment. New York: Thieme, 2006; 96-115.
72. Folmer RL, Martin WH, Shi Y, Edlefsen LL. Tinnitus sound therapy. In: Tyler RS. Tinnitus treatment. New York: Thieme, 2006;176-186.
73. Quirk WS, Avinash G, Nuttall AL, Miller JM. The influence of loud sound on red blood cell velocity and blood vessel diameter in the coch- lea. Hear Res 1992;63:102-107.
Searchfield GD. Hearing aids and tinnitus. In: Tyler RS. Tinnitus treat- ment. New York: Thieme, 2006;161-175.
Davis PB. Music and the acoustic desensitization protocol for tinnius. In: Tyler RS. Tinnitus treatment. New York: Thieme, 2006;146-160.
Björne A. Assessment of temporomandibular and cervical spine dis- orders in tinnitus patients. Prog Brain Res 2007;166:215-219.
Han BI et al.
77. Rubinstein JT, Tyler RS, Johnson A, Brown CJ. Electrical suppres- sion of tinnitus with high-rate pulse trains. Otol Neurotol 2003;24: 478-485.
78. Herraiz A, Toledano A, Diges I. Trans-electrical nerve stimulation (TENS) of somatic tinnitus. Prog Brain Res 2007;166:389-394.